|
Protocol
for Secreted Embryonic Alkaline Phosphate
1.
Intoduction
A
major area of current research in eukaryotic molecular
biology focuses on factors which control the level
of gene expression at the transcriptional and post-transcriptional
levels. One approach is to use "reporter genes" which
encode enzymes or proteins that can be quantitatively
assayed. Many genes such as B-galactosidase, galactokinase,
and CAT have been used for this purpose. Often, these
assays are expensive, time-consuming, and/or require
the use of radioactive substances. The luminescent
detection of a secreted form of human placental alkaline
phosphatase1 (SEAP), however, is an inexpensive,
fast, simple, highly-sensitive bioluminescent alkaline
phosphatase assay2.
The
Turner BioSystems TD-20/20 Luminometer combined with
CLONTECH’s Great EscAPe™ SEAP Reporter System 2 provide
a simple, sensitive method for monitoring the activity
of eukaryotic promoters and enhancers. The SEAP reporter
gene encodes a truncated form of the placental enzyme
which lacks the membrane anchoring domain, thereby
allowing the protein to be efficiently secreted from
transfected cells. Chemiluminescent alkaline phosphatase
substrate CSPD™ and an enhancer are then added to
amplify the signal, resulting in a highly sensitive
assay with linear range approximately from 10e-13
units/mL to 10e-9 units/µL alkaline phosphatase.
2.
Materials Required
- TD-20/20
Luminometer with 12 mm test tube holder and 1,000
ea 12x50 mm test tubes (P/N 2020-001)
- Adjustable
10—100 µL volume pipetter and tips
- Adjustable
1—10 µL volume pipetter and tips
- Microcentrifuge
tubes
- Centrifuge
- Deionized
water
- Great
EscAPe™ SEAP Reporter System 2 (#k2042-1) which includes:
- 10
µg of each plasmid (Basic, Enhancer, Promoter,
Control)
- 0.6
mL 25 mM CSPD™ Chemiluminescent substrate
- 12
mL Chemiluminescent enhancer
- 12
mL Assay buffer
- 5
mL 5X Dilution buffer
- 30
µL Positive control placental alkaline phosphatase
3.
Proper Use of Controls
3.1
Negative Controls - A negative control is necessary
to determine background signals from the cell culture
media. Assay 25 µL of medium from cells transfected
with the pSEAP2-Basic Vector and subtract this obtained
value from all results.
3.2
Positive Control for transfection and expression of
exogenous DNA - A positive control is used to
confirm transfection and expression of exogenous DNA
and to verify the presence of active SEAP in the culture
media. Assay 25 µL of medium from cells transfected
with the pSEAP2-Control Vector. Cells transfected
with this plasmid vector should yield greater than
100 RLU chemiluminescence within 48—72 hours after
transfection for optimum assay results. If yield is
low, increase number of cells in media. If yield is
above 5000 RLU, decrease number of cells in media
or dilute media.
3.3
Positive Control for detection method - The positive
control placental alkaline phosphatase can be used
to confirm that the detection method is working. To
do this, add 2 mL of the positive control placental
alkaline phosphatase to 23 µL of culture medium from
untransfected cells. This should give a strong positive
signal. Serial dilution of this control can be used
to determine the linear range of the assay.
Note:
Before beginning, read through entire method.
4.
Instrument Setup
4.1
Turn on instrument and allow to warm up for at
least 5 minutes.
4.2
Set instrument to:
Delay
period: 10 seconds
Integrate period: 10 seconds
Number of replicates: 1
5.
Standard Preparation
5.1
Allow a sufficient amount of Chemiluminescent
enhancer (95 µL per sample tube) and Assay buffer
(100 µL per sample tube) required for the entire experiment
to equilibrate to room temperature (20—25° Celsius).
5.2
Prepare the required amount of 1X Dilution buffer
(75 µL needed per sample tube) by adding 1 part 5X
Dilution buffer to 4 parts deionized water.
5.3
Prepare 1.25mM CSPD™ substrate by adding 1 part 25mM
CSPD™ Chemiluminescent substrate to 19 parts Chemiluminescent
enhancer. Store in the dark until use.
5.4
Add 2 µL of positive control human placental alkaline
phosphatase to 23 µL of culture medium from untransfected
cells.
5.5
Incubate for 10 minutes at room temperature.
5.6
Read standard in luminometer every 5 minutes to determine
point of maximum light emission. This is the incubation
time that should be used for the rest of your samples.
6.
Determining Linearity
6.1
Serial dilutions of the standard in step 5.6 can be
used to determine the linear range of the luminometer.
Plot [SEAP] vs. RLU on a log-log graph. On the TD-20/20,
linearity is maintained down to 10-13 units/µL
as shown in Graph 1.
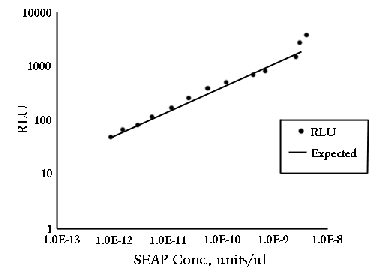
Graph 1: 2µL of positive control alkaline phosphatase
were added to 100 µL of 1.25 mM CSPD™
and incubated at room temperature. Serial dilutions
of this standard were taken to determine linear range.
7.
Transfection of Mammalian Cells with SEAP Expression
Vectors
7.1
The SEAP expression vectors may be transfected into
eukaryotic cells by a variety of techniques, including
those using calcium phosphate3, DEAE-dextran,
various liposome-based transfection reagents4,
and electroporation. A method that works well with
one cell line may not work well with another. When
working with a cell line for the first time, compare
the efficiencies of several transfection protocols
using the pSEAP2-Basic and pSEAP2-Control Vectors
as described in section 3.
7.2
Each different construct should be transfected(and
subsequently assayed) in triplicate to minimize variability
among treatment groups.
7.3
When monitoring the effect of promoter and enhancer
sequences on gene expression, it is critical to include
an internal control that will distinguish differences
in the level of transcription from variability in
the efficiency of transfection5. This is
done by cotransfecting a second plasmid which expresses
an activity different from SEAP. The level of the
second enzymatic activity can then be used to normalize
the levels of SEAP among different treatment groups.
Reporter proteins frequently used for this purpose
include E. coli b-galactosidase, which is expressed
intracellularly, and human growth hormone(hGH), which
is secreted extracellularly6.
8.
Preparation of Samples
8.1
48 - 72 hours (time for collecting samples will
vary amongst different cell types, cell densities,
and experimental conditions) after cell transfection,
remove 110 mL of cell culture medium and transfer
to a microcentrifuge tube.
8.2
Centrifuge at 12,000 x g for 10 seconds to pellet
any detached cells present in the culture medium.
8.3
Transfer 100 mL of supernatant to a fresh microcentrifuge
tube.
8.4
Store at -20° C until ready for assay.
9.
Chemiluminescent Detection of SEAP
9.1
Thaw samples of cell culture medium and place 25 µL
into a 12x50 mm polypropylene test tube.
9.2
Add 75 µL of 1X dilution buffer to sample tube. Mix
gently.
9.3
Incubate samples for 30 minutes at 65° C using a heating
block or water bath.
9.4
Cool samples by placing them in an ice bath for 2—3
minutes. Remove from ice bath and allow to equilibrate
to room temperature.
9.5
Add 100 mL of assay buffer to sample tube and incubate
5 minutes at room temperature.
9.6
Add 100 mL of 1.25mM CSPD to sample tube and incubate
until maximum signal(time is determined in step 4.9)
is achieved.
9.7
Read on TD-20/20 Luminometer.
10.
References
1.,2.
Berger, J., Hauber, J., Hauber, R., Gelger, R.,
& Cullen, B.R. © 1988 Secreted placental alkaline
phophatase: a powerful new quantitative indicator
of gene expression in eukaryotic cells. Gene 66:1-10.
3.
Chen, C. and Okayama. H. © 1988 Calcium Phosphate
mediated gene transfer: A highly efficient transfection
system for stably transforming cells with plasmid
DNA. Biotechniques 6:632-638.
4.
Kain, S.R. and Ganguly, S. © 1996 Use of secreted
alkaline phosphatase as a reporter of gene expression
in mammalian cells. Methods in Molecular Biology,
vol. 63, Humana Press, Totowa, NJ.
5.,6.
Sambrook, J. © 1989 Molecular Cloning: A Laboratory
Manual, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY.
11.
Notes
11.1
Great EscAPe™ is a trademark of CLONTECH Laboratories,
Inc.
11.2
CSPD™ is a trademark of Tropix, Inc.
|

