|
Protocol
for Alkaline Phosphatase Fluorescence
1.
Introduction
Because
of their critical functions in eukaryotic cells, methods
for measuring protein phosphatases were established
at least as early as 1953(1). In 1965 Fernley and
Walker(2) decribed the use of 4-methylumbelliferyl
phosphate (MUP) as a substrate for alkaline phosphatase.
Dephosphorylation of MUP yields a highly fluorescent
and stable product: 4-methylumbelliferone (4MU). MUP
is now widely used for phosphatase detection. In 1989
Berger(3) constructed a reporter gene, secreted embryonic
alkaline phosphatase (SEAP), in which alkaline phosphatase
is secreted from the recombinant cell. The protein
can be detected directly in the culture media with
MUP. It has also been used to detect PCR amplification
products in ELISAs and to identify and characterize
bacteria.
We
describe a method for detection of alkaline phosphatase
(AP) using MUP as a substrate and the Turner BioSystems
TD-700 Laboratory Fluorometer to measure the highly-fluorescent
enzymatic product, 4MU. The TD-700 Fluorometer enables
researchers to quantitate as little as 1 x10-7 mg/ml
(200 pg) 4MU and has a linear range of over 5 orders
of magnitude. The sensitivity of the method described
below was about 2 ug/ml alkaline phosphatase.
2.
Materials Required
- TD-700
Fluorometer with standard PMT and 10 mm × 10 mm
- square
cuvette adaptor (P/N 7000-009)
- Near
UV Lamp (P/N 10-049)
- Long
Wavelength UV Filter Kit (P/N 10-302R)
- 10
mm × 10 mm square methylacrylate disposable cuvettes
(P/N 7000-959)
- 4-Methylumbelliferone
(MU), sodium salt, F.W. 198.2
- 4-Methylumbelliferyl
phosphate (MUP), free acid, MW 256
- Alkaline
Phosphatase Standard
- Sodium
Carbonate, Anhydrous Na2CO3,
MW 106.0
- 50mM
Tris Buffer pH 8.0
- Bovine
Serum Albumin (BSA)
3.
Experiment Protocol
3.1
Reagent Preparation
4MU
Stock Solution, 50 mM. Dissolve 99.1mg of 4MU
in 10 mL deionized water. Store at 4°C in the dark
for up to two months.
4MU
Standard Solution, 10 uM. Dilute 10 uL 4MU Stock
Solution into 50 mL Tris/0.1% BSA Buffer, pH 8.0.
Store at 4°C in the dark for up to two months.
Sodium
Carbonate Solution, 0.2 M. Dissolve 2.12g Na2CO3
into 100 mL distilled water. pH is approximately
12.
MUP
Substrate Stock Solution, 3.6 mM. Dissolve 9.2
mg MUP into 10 mL 50 mM Tris/0.1% BSA buffer, pH
8.0. Make up fresh daily. NOTE: MUP spontaneously
hydrolyzes in aqueous solution. It should be stored
in its solid form and made up just prior to use.
MUP
Substrate Working Solution, 36 uM. Dilute 300
uL MUP Substrate Stock Solution into 30 mL Tris/0.1%
BSA Buffer, pH 8.0. Make up fresh daily.
Alkaline
Phosphatase Stock Solution, 1 mg/mL. (Biozyme
calf-intestine alkaline phosphatase, 15.42 mg/ml).
Dilute 100 ul alkaline phosphatase into 1.4 mL Tris/0.1%
BSA buffer, pH 8.0.
Alkaline
Phosphatase Standard Solution, 500 ug/mL. Dilute
1ml AP Stock Solution with 1mL Tris/0.1% BSA buffer.
3.2.
Instrument Set-Up
- Turn
on the TD-700 Fluorometer. Allow it to warm up for
10 minutes (600 seconds).
- Ensure
the lamp is installed by checking that the small
window in the back panel is lit or by removing the
filter cylinder and observing the lamp emission
in the sample chamber.
-
Ensure that the excitation filter, P/N 10-069R,
is installed in the position marked A"EX", and the
emission filter, P/N 10-110R-C, is installed in
the corresponding position marked A"EM" in the filter
cylinder. Install the filter cylinder so the A is
next to the silver dot on the left.
3.3.
Instrument Calibration
- Prepare
the 1 uM 4MU calibration solution by adding 0.5
ml 36 uM MUP solution into a 10x10 mm cuvette. Add
0.25ml 10uM 4MU Standard Solution and 1.75ml sodium
carbonate solution. Cover and mix by inversion.
- Place
the cuvette in the 10 x 10 mm cuvette sample adapter.
Place the sample adapter into the sample compartment.
Be sure the pointed end of the sample adapter handle
(it has a dot on it) is directed to the left and
toward the letter on the filter cylinder.
- Calibrate
the instrument with the 1.0 uM 4-MU Standard solution
in the multi-optional mode according to the TD-700
Operating Manual, p. 21. Set the ‘Sample Setting’
to 900. Choose ‘9’ or ‘No’ when prompted ‘Read &
Subtract Blank?’.
3.4
Alkaline Phosphatase Standard Curve
- To
generate a single-replicate, six-point standard
curve from 20 ug/mL to 1 ug/mL, add 0.5 mL 36 uM
MUP Working Solution to each of 6 cuvettes.
- Add
an aliquot of 500 ug/mL AP Standard Solution to
a cuvette and incubate the mixture for two minutes
at room temperature.
- Add
Sodium Carbonate Solution to the cuvette to make
a total volume of 2.5 mL. Mix.
- Take
a fluorescence measurement immediately.
- Repeat
steps 3.4.1 through 3.4.4 with each standard (Table
1).
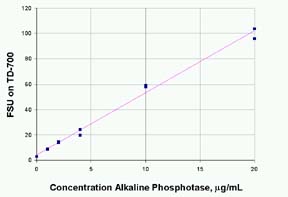
- Generate
a standard curve of fluorescence versus alkaline
phosphatase concentration. Shown in Figure 1 below
is the fluorescence of 4MU from reaction of alkaline
phosphatase with 36 mM methylumbelliferone phosphate
(MUP) and quenched with 100mM Na2CO3.
The standards were run in duplicate. The R2 is 0.992.
The fluorescence value of the reagent blank may
be subtracted from that of each sample.
Table
1
|
Volume (ul) of 36uM
MUP working solution
|
Volume (ul) of 500ug/ml
AP standard
|
Volume (ml) of
Na2CO3 Solution
|
Final AP concentration
in standard solution (ug/ml)
|
|
500
|
100
|
1.90
|
20
|
|
500
|
50
|
1.95
|
10
|
|
500
|
20
|
1.98
|
4
|
|
500
|
10
|
1.99
|
2
|
|
500
|
5
|
2.00
|
1
|
|
500
|
0
|
2.00
|
0
|
Figure
1 - Standard Curve
3.5
Alkaline Phosphatase Samples
- Add
0.5 mL 36 uM MUP Working Solution to each sample
cuvette.
- Add
100 uL of sample to a cuvette, invert to mix.
- Incubate
the mixture for two minutes at room temperature.
- Add
1.9 mL Sodium Carbonate Solution to the cuvette
to make a total volume of 2.5 mL. Mix.
- Take
a fluorescence measurement immediately.
- Repeat
with each sample.
- Calculate
the amount of alkaline phosphatase from the fluorescence
measurement and the linear equation determined from
the AP standard concentration vs. fluorescence,
step 3.4.6.
4.
Discussion
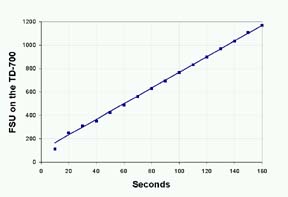
Alkaline
phosphatase kinetics can be measured on the TD-700
using the Data Stream feature. Press the 7 key and
choose (Data Stream) Figure 2 shows an example of
the reaction data. Reagent concentrations and reaction
times can be optimized using this feature.
Figure
2: Real-time Fluorescence of 4MU from reaction of
1ug
Alkaline Phosphatase in 36 uM Methylumbelliferone Phosphate
(MUP).
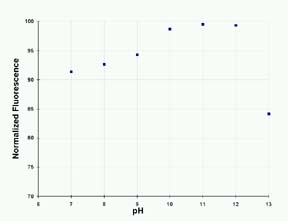
The
effect of pH on 4MU fluorescence is shown in Figure
3. The enzymatic reaction proceeds best at a pH of
about 8; the optimum pH for 4MU fluorescence is 10
to 12.
Figure
3: The Effect of pH on the Fluorescence of 4-MU
in 200 mM Sodium Carbonate Solution.
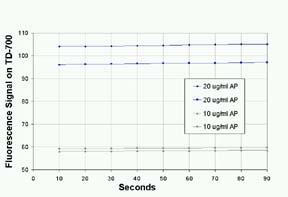
For
quantitating enzyme, a stopped-reaction method is
faster than a direct-initial reaction rate method.
However, the accuracy of the method depends on precise
timing and how well the reaction is quenched by the
stop solution. The addition of the sodium carbonate
solution slowed the reaction rate to less than 2%.
Duplicate results with AP standards at two concentrations
are shown in Figure 4.
Figure
4: Stability of Fluorescence of Alkaline Phosphatase
with MUP after Quenching with Sodium Carbonate, pH12.
Guilbault(4)
found that as little as 10-6 units/ml of alkaline
phosphatase could be measured with about 1.5% accuracy
by a direct initial reaction rate method using 20
mM MUP. He found that a 1.7 mM concentration of phosphate
caused 50% inhibition and measured the effects of
several other enzymes including ß-glucosidase on the
alkaline phosphatase reaction. Fernley and Walker(2)
reported the effects of various reaction conditions.
They used 0.5M K2HPO4-KOH buffer, pH 10.4 to stop
the reaction. They note that the addition of 5mM magnesium
chloride increased the activity of the enzyme by up
to 100%, but activity varied with both MgCl2 and MUP
concentration. Several other enzymes can be measured
using the fluorescence of 4MU derivatives. These include
acid phosphatase, ß-D-galactopyranoside, a-D-glucopyranoside,
ß-D-glucopyranoside, ß-D-glucuronidase, and lipase.
5.
References
- Brandenberger,
H., and Hanson, R., Spectrophotometeric Determination
of Acid and Alkaline Phosphatases, Helv. Chim. Acta,
36, 900, 1953.
- Fernley,
H. N. and Walker, P. G., Kinetic Behaviour of Calf-Intestinal
Alkaline Phosphatase with 4-Methylumbelliferyl Phosphate,
Biochem. J., 97, 95, 1965.
- Berger,
J., et. al., Secreted Placental Alkaline Phosphatase:
a Powerful New Quantitiative Indicator of Gene Expression
in Eukaryotic Cells, Gene, 66, 1, 1988.
- Guilbault,
G. G. and Sadar, S. H., Umbelliferone Phosphate as
a Substrate for Acid and Alkaline Phosphatase, Analytical
Letters, 1(5), 333, 1968.
6.
Nomenclature
4-methylumbelliferone
is listed in the Merck Index as Hymecromone with the
following synonyms: 7-hydroxy-4-methyl-2H-1-benzopyran-2-one,
7-hydroxy-r-methylcoumarin, 4-methylumbelliferone,
ß-methylumbelliferone, and 4-MU. The free acid is
C10H8O3, MW 176.2. Its form is off-white or yellowish
crystals or powder. It is soluble in MeOH and EtOH,
has blue fluorescence in alcohol and water, and is
practically insoluble in cold water at neutral pH.
The sodium salt, C10H7O3Na, MW 198.2, is a yellow
crystalline powder and is freely soluble in water.
Molecular Probes lists the free acid as 7-hydroxy-4-methylumbelliferone.
It is 4-methylumbelliferone (free acid or sodium salt)
in the Sigma Chemical catalog.
|

