|
Protocol
for ENLITEN« ATP Assay System Bioluminescence Detection
Kit for ATP
1.
INTRODUCTION
The
Turner BioSystems TD-20/20 Luminometer combined with
the Promega ENLITEN« ATP Assay System Bioluminescence
Detection Kit provides a sensitive, rapid method for
measuring adenosine 5'-triphosphate (ATP). A reliable
method for ATP detection is useful for studying enzymes
that produce or degrade ATP. ATP detection also provides
an indirect measurement of microbes, food residue, or
other biological material.
The
ATP-dependent oxidation of luciferin by luciferase produces
light. When ATP is the limiting factor in the luciferin
oxidation reaction, the amount of light produced is
proportional to the ATP concentration of the sample.
The
highly sensitive TD-20/20 can detect attomole levels
of ATP. The limit of detection for ATP with the ENLITEN«
detection assay is 1x10-16 moles (Figure
1).
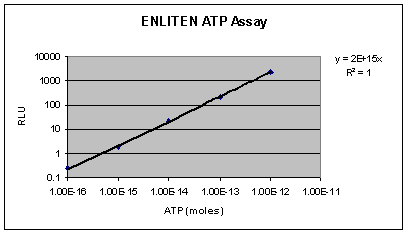
Figure
1. A standard curve obtained using the TD-20/20
Luminometer and the ENLITEN« ATP Assay System.
10 ÁL of ATP Standard diluted in HEPES buffer was added
to a test tube containing 100 ÁL rL/L Reagent.
When
designing your ATP assay with the ENLITEN«
kit, it is important to remember several key aspects
of the luciferase reaction. First, the rL/L reacts optimally
at a pH of 7.73 and 23Ś25░C. Salts and many nonionic
chemicals will impair light production. Therefore, it
is necessary to take care when selecting buffers and
ATP extractants for the sample preparations. It is also
recommended to check for ATP contamination in your assay
buffer by comparing the RLU values obtained with your
assay buffer and rL/L to those of ATP-Free Water.
Within
your sample, there may exist several different ATP stores.
For example, in cell preparations, ATP may be present
in the media. Treatment of cells may alter the amount
of ATP in the media.
If
you wish to measure the ATP content in microorganisms
or cells, you will need to extract the ATP before analysis.
Trichloroacetic acid (TCA) is recommended because it
releases ATP from cells and inactivates ATP-degrading
enzymes. Because TCA inhibits the luciferase reaction,
it is important to determine the minimum amount of TCA
necessary. Generally, 0.5% to 2.5% TCA (final concentration)
is sufficient for ATP extraction from bacteria and eukaryotic
cells.
Preparing
a standard curve is a useful tool for proper ATP analysis
of your samples. A standard curve should be prepared
daily or whenever a new aliquot of the rL/L Reagent
is used. The standard curve should include varying concentrations
of ATP diluted in your assay buffer. The composition
of this buffer should be identical to the composition
of the buffer used in your assay to prepare your samples.
Keeping
these considerations in mind will help you obtain the
most accurate ATP analysis.
2.
MATERIALS REQUIRED
From
Turner BioSystems:
- TD-20/20
Luminometer (P/N 2020-000)
- 12
mm Test Tube Holder (P/N 2020-950)
- 12
x 50 mm Polypropylene Test Tubes (P/N 2020-955)
From
Promega:
- ENLITEN«
ATP Assay System Bioluminescence Detection Kit for
ATP (P/N FF2000)
- Contains:
- 1
vial rLuciferase/Luciferin (rL/L)Reagent
- 12
mL Reconstitution Buffer
- 1
vial ATP Standard (1x10-7 M)
- 25
mL ATP-free Water
Other
Materials:
- Adjustable
p1000 Volume Pipetter and Tips
- Adjustable
p200 Volume Pipetter and Tips
- Assay
buffer
- Test
tube rack compatible with 12 x 50 mm polypropylene
tubes
- Nitrile,
vinyl, or latex gloves
Storage
Conditions: The rL/L Reagent and Reconstitution
Buffer must be stored at -20░C prior to reconstitution.
Store the ATP Standard at -20░C.
Note:
Individuals sensitive to latex should use vinyl or
nitrile gloves.
3.
REAGENT AND STANDARD PREPARATION
Note:
ATP contamination will cause erroneous results and
increase background. Wear gloves to prevent ATP
contamination from your hands during reagent preparation
and while performing the assay.
3.1
Equilibrate the sample buffer to room temperature.
3.2
Lightly tap the vial of the rL/L Reagent before opening
to ensure the lyophilized material collects at the
bottom of the vial.
3.3
Transfer the contents of the vial of rL/L Reconstitution
Buffer to the vial of the rL/L Reagent.
3.4
Replace the stopper and slowly invert the vial several
times to dissolve the contents. Do not shake the reagent
bottle.
3.5
Allow the reconstituted rL/L Reagent to stand at room
temperature for 1 hour before use.
Note:
Reconstituted rL/L Reagent may be kept for 8 hours
at room temperature. Store at -20░C in single-use
aliquots for long-term storage.
3.6
Prepare a 10-fold serial dilution of the ATP standard
(1x10-7 M) assay buffer in labeled test
tubes. Dilute to 1x10-11 M ATP.
4.
INSTRUMENT SET-UP
4.1
Turn on instrument and allow to warm up for at least
5 minutes (300 seconds).
4.2
Choose the Standard (STD) Mode and adjust settings
so that:
Delay
period = 2 sec.
Integrate period = 10 sec.
Replicates = 1
5.
ATP STANDARD CURVE PROCEDURE
5.1
Prepare several test tubes with 100 ÁL of rL/L Reagent
only.
5.2
Press <0> on the luminometer to begin the blank
subtraction sequence.
5.3
Add 10 ÁL of assay buffer before pressing for
each blank sample.
5.4
The luminometer will read and record the values for
each blank sample. These values will be averaged and
recorded as the background level.
5.5
Press <1> to accept the background level. This value
will now be subtracted from subsequent measurements.
Note:
It is important to determine the background level
of ATP due to contamination. The blank subtraction
should be performed each day the ATP assay is run.
5.6
From the dilutions obtained in step 3.6, add 10
ÁL of the lowest concentration (1x10-11
M) of ATP to a labeled test tube containing 100 ÁL
rL/L Reagent.
5.7
Place the labeled test tube into the TD-20/20
Luminometer and close the lid.
5.8
Press to begin measurement. After the 2 second
delay, the instrument will begin the 10-second measurement
period.
5.9
Record this value and repeat with the next concentration
of ATP. Continue for a total of five concentrations
from 1x10-11 to 1x10-7 M ATP.
6.
SAMPLE ANALYSIS
6.1
Add 10 ÁL of your sample prepared in the assay
buffer to a labeled test tube containing 100 ÁL rL/L
reagent.
6.2
Press . After the 2-second delay, the instrument
will begin the 10-second measurement period.
6.3
Record this value and repeat steps 6.1 - 6.2 for
your remaining samples.
6.4
Plot the RLU values of your samples along with the
RLU values obtained during the standard curve procedure
to determine the concentration of ATP in your samples.
7.
ABOUT PROMEGA CORPORATION
Orders
for Promega's products may be placed by:
Toll-Free:
(800) 356-9526
Fax: (800) 356-1970
Email: custserv@promega.com
Mailing
Address (HQ):
Promega Corporation
2800 Woods Hollow Rd.
Madison, WI 53711 USA
|

